Nitric acid, also known as aqua fortis, is a highly corrosive and toxic acid that is widely used in different industries. It is a colorless or yellowish liquid that has a pungent odor and can cause severe burns upon contact with skin. Nitric acid is an important chemical compound that plays a significant role in the manufacturing of various products, including fertilizers, dyes, and explosives.
In this article, we will explore what nitric acid is and how it is used in different industries.
What is Nitric Acid?
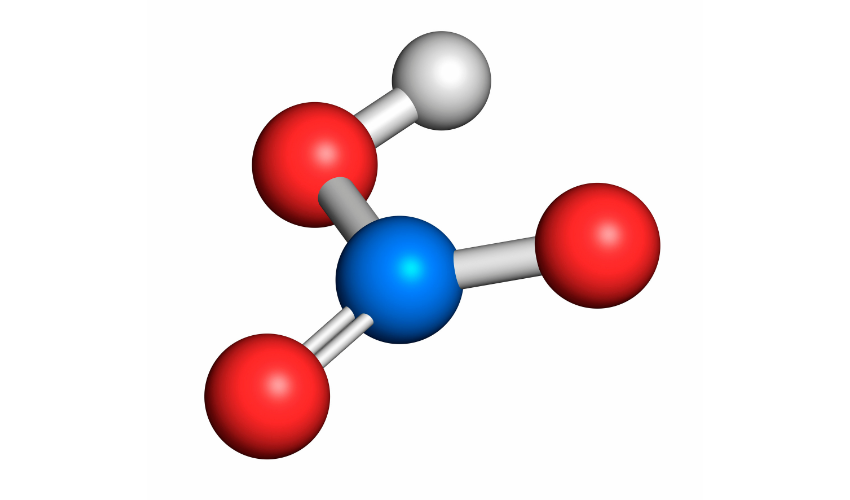
Nitric acid is a highly reactive compound that contains nitrogen, oxygen, and hydrogen. It has the chemical formula HNO3 and is a strong oxidizing agent. It is soluble in water, and the resulting solution is highly acidic with a pH of approximately 1.
Nitric acid is produced by the reaction of nitrogen dioxide with water, as shown below:
NO2 + H2O → HNO3
Nitric acid is commonly available in different concentrations, ranging from 50% to 70%. It can also be synthesized by the oxidation of ammonia in the Ostwald process.
What is Nitric Acid Used For?

Nitric acid finds its application in various industries, including:
- Pharmaceuticals: It is used in the production of certain drugs, such as nitroglycerin, which is used to treat heart diseases.
- Fertilizers: It is used in the manufacturing of nitrogen-based fertilizers, such as ammonium nitrate, which is widely used in agriculture.
- Explosives: It is a key component in the production of explosives, such as trinitrotoluene (TNT) and nitroglycerin.
- Dyes: It is used in the manufacturing of dyes and pigments.
- Metallurgy: It is used in the etching and cleaning of metals, such as copper and brass.
- Cleaning agents: It is used in the manufacturing of cleaning agents, such as toilet bowl cleaners and metal cleaners.
- Food processing: It is used as a food additive in the production of cheese and other dairy products.
- Photography: It is used in the development of photographic films and plates.
- Water treatment: It is used in the treatment of water to remove bacteria and other harmful contaminants.
How is Nitric Acid Used?
Nitric acid is a highly reactive acid that should be handled with care. It can cause severe burns upon contact with skin and can also be toxic if ingested or inhaled. The following are some common uses of nitric acid:
- Etching: It is used to etch metals, such as copper and brass, by dissolving the unwanted portions of the metal.
- Cleaning: It is used as a cleaning agent to remove stains and rust from metal surfaces.
- Explosives: It is used in the production of explosives, such as TNT and nitroglycerin.
- Fertilizers: It is used in the manufacturing of nitrogen-based fertilizers, such as ammonium nitrate.
- Medical applications: It is used in the production of certain drugs, such as nitroglycerin, which is used to treat heart diseases.
FAQs
What are the dangers of handling nitric acid?
It is a highly corrosive and toxic acid that can cause severe burns upon contact with skin. It can also be toxic if ingested or inhaled. Proper precautions should be taken when handling nitric acid, including wearing protective clothing and gloves, and working in a well-ventilated area.
Is nitric acid safe for food processing?
It is safe for food processing when used in small quantities as a food additive. It is used in the production of certain cheeses and other dairy products.
How is nitric acid produced?
It is produced by the reaction of nitrogen dioxide with water or by the oxidation of ammonia in the Ostwald process.
What is the concentration of nitric acid used in industrial applications?
It is commonly available in concentrations ranging from 50% to 70%. The concentration used in industrial applications depends on the specific use.
What is the role of nitric acid in the production of explosives?
It is a key component in the production of explosives, such as TNT and nitroglycerin. It is used in the nitration process to introduce nitro groups into organic compounds.
Conclusion
Nitric acid is a highly reactive and corrosive acid that finds its application in various industries. It is used in the production of drugs, fertilizers, explosives, dyes, cleaning agents, and food additives. Nitric acid is also used in metallurgy, photography, and water treatment. Although nitric acid is a powerful chemical, it should be handled with care and used only in appropriate quantities.